Medical Device Reimbursement in the USA
Medical Device Reimbursement in Germany
Medical Device Reimbursement in France
Medical Device Reimbursement in the UK
Summary: Avoiding Medical Device Reimbursement Failure
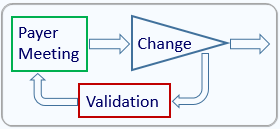
We have seen cases where a payer's feedback convinced the company to modify its selected comparator, changed the study's patient population and even its end points, in order to better address the requirements for reimbursement. Learning about payers' requirements early on, prior to initiating a clinical study, may save the company from having to conduct another study, post marketing clearance/approval, just to address payers' needs, significantly lowering costs and shortening the time to market.
Mediclever would be happy to guide you through these pathways in the above mentioned countries and others.