Navigating the Global Market Access Landscape
Background
During the last 20 years, we have worked with more than 200 medical device companies on the reimbursement of their innovative medical devices.
This article shares five tips that could help medical device startups avoid costly mistakes when planning for the reimbursement of their devices. These tips are relevant for companies planning to launch their devices in Europe, the US or both.
Tip #1: Determine the setting in which your device will be used
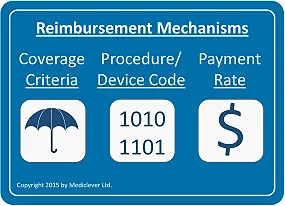
Reimbursement mechanisms are comprised of:
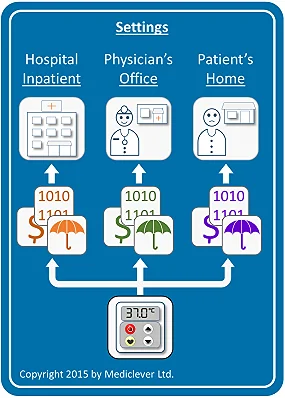
If the same device is used in the physician's office setting, it might be covered and paid for as a German Einheitlicher Bemessungsmassstab (EBM) item, a French CCAM item, or funded through individual Clinical Commissioning Groups' (CCGs') or local authorities' budgets in the UK.
Finally, if the same device is used in the patient's home, it might be covered and paid for as an item in the German Resource Directory (hilfsmittelverzeichnis), the French List of Reimbursable Products and Services (LPPR) or funded by the local UK CCG, and in some cases, as an item in Part IX of the Drug Tariff.
Therefore, the first step in planning for reimbursement is identifying the setting or settings in which your device will be used.
Tip #2: Determine whether your device could utilize existing reimbursement mechanisms
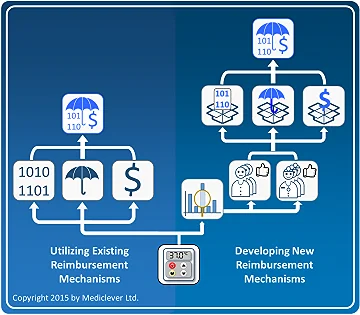
After determining the relevant setting or settings in which your device will be used, you will need to clarify whether your device could utilize any existing reimbursement mechanisms in those settings. There is a big difference between obtaining reimbursement by utilizing existing reimbursement mechanisms (coverage criteria, procedure/device codes, payment rates) which may be used to bill for your device, and obtaining reimbursement by developing new reimbursement mechanisms to suit your new device.
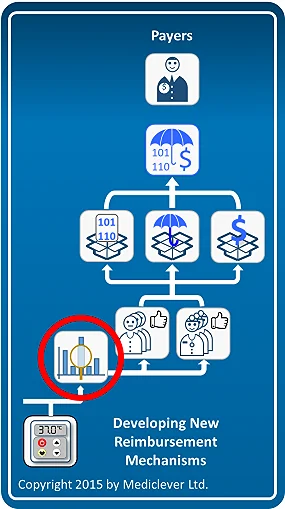
As shown in the left column of the image on the right, obtaining reimbursement by utilizing existing reimbursement mechanisms is almost immediate. Once the applicability of these existing reimbursement mechanisms has been verified, healthcare providers may bill for the use of your device and get reimbursed according to them.
The process and timeline of obtaining reimbursement by developing new reimbursement mechanisms is very different. As shown in the right column of the image on the right, obtaining reimbursement by developing a new code, new coverage criteria, and/or a new payment rate, requires the development of specific clinical data to prove the clinical and economic benefits associated with the use of your device, as well as being able to establish an initial user base in each country and harness the support of the local medical community. This process could take years and add significant costs to your commercialization plan. In some cases, companies may consider changing a feature in their device or tweaking it in some way, just in order to fit under existing reimbursement mechanisms.
Therefore, whenever possible, try to identify early on whether your device could utilize existing reimbursement mechanisms that would facilitate an easier, quicker, and cheaper commercialization pathway.
Tip #3: Focus on your main decision maker
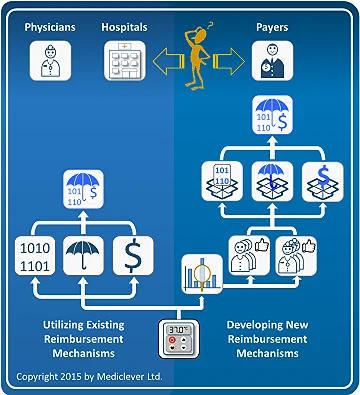
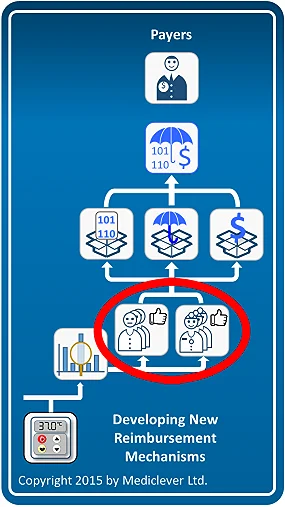
One of the derivatives of determining whether your device can utilize existing reimbursement mechanisms or requires the development of new ones, is identifying the main decision maker for the commercialization of your device.
If your device can utilize existing reimbursement mechanisms (left column), payers do not have to be involved. Therefore, physicians and hospital management become your main decision makers. Once you are able to convince physicians and hospital management (in case the device is used in the hospital inpatient setting) of the clinical and economic benefits of using your device instead of the currently available alternatives, hospitals will purchase your device and bill for it under existing reimbursement mechanisms.
The same applies for physicians only, when the device is used in the physician's office setting or the patient's home setting.
On the other hand, if new reimbursement mechanisms need to be developed (right column), convincing physicians and hospitals will not be enough.
Payers will have to be convinced of the clinical and economic benefits of using your device instead of the currently available alternatives before they agree to develop the required reimbursement mechanisms and facilitate device commercialization. Typical decision makers in Germany may be the Institute for the Hospital Payment System (InEK) for devices used in the hospital inpatient setting, the Federal Joint Committee (G-BA) for devices used in the physician's office, or the Association of Statutory Health Insurance (SHI) sickness funds (GKV-Spitzenverband) for devices used in the patient's home. Similarly, typical decision makers in France may be the National Commission for the Evaluation of Medical Devices and Health Technology (CNEDiMTS), the National Union of Health Insurance Funds (UNCAM), and the Economic Committee for Health Products (CEPS).
Therefore, after clarifying your main decision makers, all subsequent steps should be conducted from your main decision makers' perspective. Whereas the physician or hospital may be interested in the clinical benefit for their specific patients, a payer may have a more holistic view concerning the clinical benefit for all of its beneficiaries. Similarly, economic benefits as seen by the physician or hospital may be different from the payer's perspective. As a result, clinical studies should be designed with the main decision maker's perspective in mind and any developed economic models should be done from the point of view of your main decision maker.
Tip #4: Think of reimbursement when formulating your clinical development plan
Therefore, in order to avoid unnecessary delays and costs, try to leverage your planned clinical studies to also generate the data required by reimbursement authorities later.
Tip #5: Plan for harnessing the support of the local medical community and your patients
In other countries regional hospital grants (e.g., Italy and Spain) or even support from charity funds (e.g., the UK) may be utilized to help with establishing this initial user base.
Establishing an initial user base for devices used in other settings may be more challenging, although some local payers may be willing to initiate and partially fund pilot projects, testing the new device in a real-world scenario. Recently, a new "Innovation Fund", which provides funding for particular projects, was established in Germany. Funding from this Innovation Fund may also be used to generate required evidence and help in establishing an initial user base.
Therefore, companies should plan in advance to leverage the above mentioned methods in order to establish an initial user base and harness the support of the local medical community to facilitate sales before the device secures formal reimbursement.
Summary
We hope that the above tips would be of value to anyone who plans to commercialize a medical device. If you have any reimbursement related questions, or would like to speak with one of our consultants, just let us know.
Mediclever can assist by clarifying the relevant settings for your device, determining whether any existing reimbursement mechanisms may be utilized in each setting and identifying the main decision makers. Then, we will help you formulate your clinical development plan and economic model from your main decision makers' perspective and work with you to establish a documented user base and harness the support of the local medical community by leveraging our contacts in North American and European healthcare markets.
Frequently Asked Questions About Medical Device Reimbursement
How long does medical device reimbursement take?
Medical device reimbursement timelines vary by country and care setting. Devices that can use existing reimbursement mechanisms may achieve reimbursement within months, while developing new reimbursement codes, coverage criteria, and payment rates can take several years.
Who decides medical device reimbursement in Europe?
Medical device reimbursement decisions in Europe depend on the country and care setting and may involve payer organizations, health technology assessment bodies, and national reimbursement authorities.
Can medical device startups use existing reimbursement codes?
Yes. Many medical device startups can commercialize their devices faster by using existing reimbursement mechanisms rather than developing new reimbursement codes and coverage criteria.
What factors affect medical device reimbursement in different countries?
Reimbursement for medical devices depends on the care setting, existing coverage mechanisms, national regulations, payer requirements, and the clinical and economic evidence supporting the device.
How can startups accelerate reimbursement for their medical devices?
Startups can accelerate reimbursement by leveraging existing reimbursement mechanisms, engaging key decision makers early, planning clinical studies with reimbursement in mind, and building an initial user base with support from the medical community.