Why a Medical Device Reimbursement Strategy is Critical
Understanding the Risks of Misaligned Strategies
Many companies pursue various reimbursement activities without first establishing a clear medical device reimbursement strategy, resulting in misaligned coding, coverage, and payment efforts that fail to achieve reimbursement.
Over the last 20 years, we have helped more than 200 medical device companies worldwide develop and implement effective medical device reimbursement strategies. In many cases they approached us after they had already tried to develop a code, apply for coverage, or pursue various reimbursement achievements.
However, we often learned that they had been barking up the wrong tree, since the reimbursement achievements they pursued could not have helped them achieve their goal of obtaining reimbursement for their product.
Suggested reimbursement approach
The purpose of this article is to guide companies on how to develop a reimbursement strategy that would help them focus on pursuing the specific reimbursement achievements that would lead to product reimbursement.
This approach has been consistently validated during the last decade and has shown to help pharma and medical device companies develop reimbursement strategies that eliminated waste of time and resources and facilitated favorable reimbursement in various markets.
It should be noted that the process below is focused on the development of a strategy (the "what" we should do). It does not deal with the implementation (or the "how" to do it part). To learn about the implementation of the reimbursement strategy (or the "how" to do it part), please refer to the articles on the right:
Suggested reimbursement strategy development process
Here’s a step-by-step approach to determining whether similar devices are already reimbursed and how to plan your strategy accordingly.
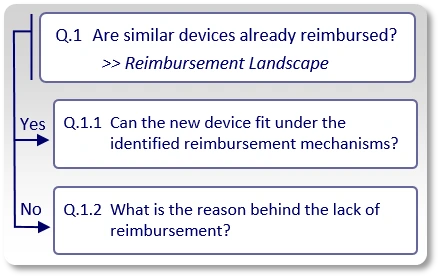
The first question to answer (Q.1) is whether similar or comparable devices are already reimbursed. In order to answer this question we conduct our Activity 1, also known as "Reimbursement Landscape" in which we indicate:
- Which specific codes may be used to bill for similar products?
- Applicable coverage restrictions. Do payers restrict coverage for similar devices to certain criteria (e.g., only if treatment X has already failed), indications (e.g., only for the treatment of disease A, but not disease B), or certain sub-segments of the population (e.g., only patients above a certain age or with certain comorbidities)?
- What is the assigned payment rate for the use of similar devices?
Your reimbursement strategy depends on the answer
Whether similar devices are already reimbursed determines who the decision makers are and which reimbursement pathway should be pursued.
Commercialization under existing reimbursement mechanisms
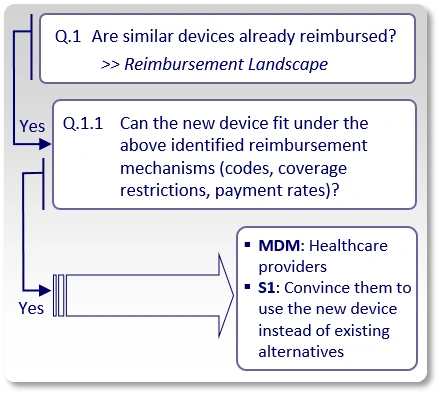
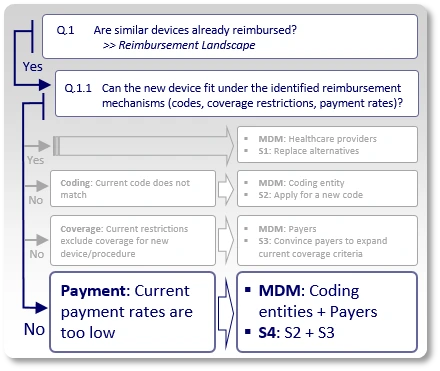
If the answer to "Q.1" is "Yes", meaning, we found existing reimbursement mechanisms (applicable codes, coverage policies and payment rates) for similar devices, we ask whether or not the new device can actually be commercialized under those existing reimbursement mechanisms (Q.1.1).
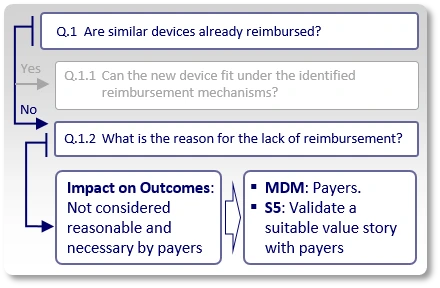
If the answer to "Q.1" is "No", meaning, we could not find existing reimbursement mechanisms (applicable codes, relevant positive coverage policies and applicable payment rates) for similar devices, we need to understand the reason for this lack of reimbursement (Q.1.2).
Let's start with Q.1.1.
Since there are existing reimbursement mechanisms for similar devices, we try to clarify whether the new device can actually be commercialized under those identified existing reimbursement mechanisms. In case the answer to Q.1.1 is "Yes", there is no need to seek reimbursement and to approach payers. The main decision makers (MDMs) are the healthcare providers (hospitals, physicians) who currently use such devices and should be convinced to start using the new device, under the existing reimbursement mechanisms, instead of the currently available devices.
The strategy (S1) shifts from payer advocacy to a Value-Based Procurement strategy. All clinical data and economic models must be tailored to satisfy Hospital Value Analysis Committees (VACs) rather than national payers. All claims, clinical data, economic models, etc., should be developed from the perspective of healthcare providers, and used to convince them (rather than payers).
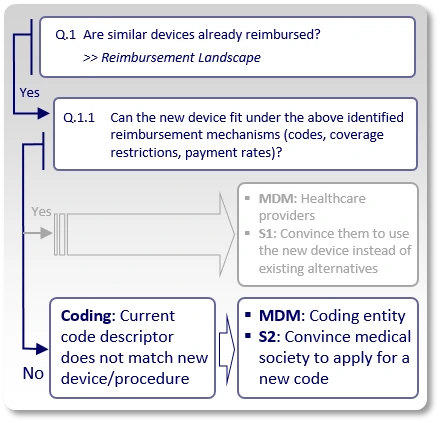
If the answer to Q.1.1 is "No", we need to understand the reason. In many cases, the reason is one of the following:
Strategy S2: Coding Considerations
Similar devices are reimbursed, but their assigned code excludes the use of the new device. For example, the assigned code's descriptor indicates a specific technology (e.g., pneumatic, electric, mechanic), certain features (number of channels, pressure ranges), material (type, size, attributes) and so on, that are not utilized/offered by the new device. This means healthcare providers can not report the existing identified code when using the new device.
In such case, the strategy (S2) should aim to modify the existing code's descriptor or create a new code with a descriptor that describes the use of the new device. The main decision makers (MDMs) are the coding entities (such as the AMA in the US), in charge of modifying/creating new codes.
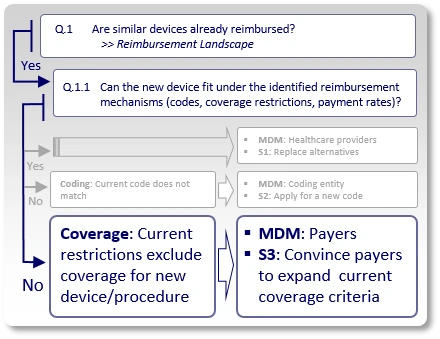
Strategy S3: Coverage Considerations
Similar devices are reimbursed, and existing codes may even apply to the use of the new device, but payers have issued coverage policies that inhibit coverage for the new device by excluding certain technologies (e.g., pneumatic, electric, mechanic), applications (covered for treatment/diagnosis of X, but not for Y), treated sub-segments of the population (covered for adults, but not for children) or applying any other restrictions that keep the new device outside existing coverage criteria.
In such case, the strategy (S3) should aim to modify and expand current coverage criteria to allow inclusion of the new device's characteristics under those payers' coverage policies. The main decision makers (MDMs) are obviously payers.
Strategy S4: Payment Considerations
In case the new device fits under the descriptor of an existing code and can be used under existing coverage criteria, but the current payment rate, which is assigned to the existing code, is too low, the company may:
- Change the price of the new device to reflect current market conditions and commercialize it under the identified existing reimbursement mechanisms (code, coverage policy and payment rate), or:
- Develop new reimbursement mechanisms for the new device. This would require:
- Convincing the relevant coding entity that the new device is substantially different than the existing ones and the current code that describes those similar devices, cannot be used to describe the new device, plus:
- Following strategies S2 and S3 described above to convince the relevant coding entity to develop a new code for the new device and convince payers to cover it.
From our experience, if similar devices are already reimbursed and your new device can not fit under those existing reimbursement mechanisms, the reasons are usually one of the above. However, there might be other reasons, which will have to be analyzed to enable the development of an appropriate reimbursement strategy.
Commercialization through the development of new reimbursement mechanisms
Now, let's see what happens if the answer to question "Q.1" is "No", meaning we could not find existing reimbursement mechanisms (applicable codes, relevant positive coverage policies and applicable payment rates) for similar devices.
First, it is important to understand the reason for this lack of reimbursement (Q.1.2). In many cases, the reason is one of the following:
Impact on outcomes:
In some cases, payers may argue that current devices' impact on clinical outcomes does not justify reimbursement. For example, if the use of similar devices enables patients to walk faster, improve their grip or engage in certain sporting activities, payers may argue that it is unreasonable for them to pay for these achievements.
In such case, the strategy (S5) is to validate with a few payers, in advance, that should the company's planned clinical studies (whose protocol synopses are shown in advance to these payers) generate the expected results, payers would consider the new device to provide a sufficient positive impact on medical outcomes, which justifies reimbursement.
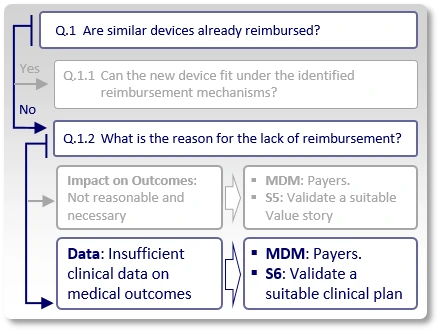
Data:
Payers may agree that in theory, similar devices may have sufficient positive impact on medical outcomes, but they do not reimburse them since the peer-reviewed, published literature does not currently provide the evidence to support it.
Preferably, the company's data should demonstrate that:
- The use of the new device leads to different medical decision making,
- The different medical decision leads to a corresponding different diagnosis/treatment, and
- The difference in diagnosis/treatment leads to an improvement in clinical outcomes.
In such case, the strategy (S6) is to review similar devices' published clinical data, improve clinical study design and validate with a few payers, in advance, that should the company's planned clinical studies (whose protocol synopses are shown in advance to these payers) generate the expected results, payers would consider them as sufficient evidence to support the claimed benefits, which justify reimbursement.
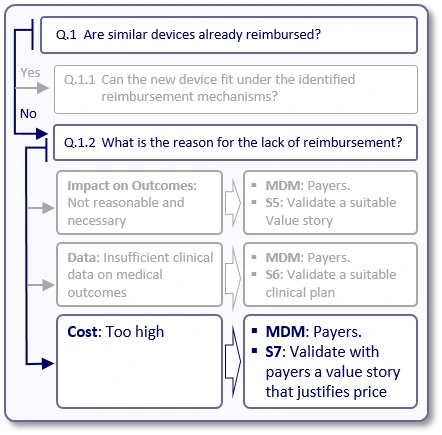
Cost:
Although payers will seldom admit it, in some cases they may refuse to reimburse new devices since their price is considered too high for the estimated generated value.
In such case, the strategy (S7) should aim to justify the price by demonstrating superior value compared with the current, non-reimbursed devices, or simply, lower costs.
In 2026, this increasingly involves Total Cost of Care (TCOC) modeling, demonstrating that a higher device price is offset by a reduction in long-term complications or hospital readmissions.
Obviously, there may be additional reasons for the lack of reimbursement that have not been reviewed here. For example, there may be no reimbursement for similar or comparable products since there are no such products available. In such case, follow strategies S5, S6 and S7.
In any case, at the basis of any reimbursement strategy there must first be a clear understanding for the reasons that inhibit current reimbursement. Only then can the company plan its next activities to promote obtaining reimbursement for its new device.
Summary and Next Steps
If you are developing a new device or correcting a delayed reimbursement pathway, our medical device reimbursement strategy services can help align your evidence, decision makers, and commercialization plan.
Pursuing different reimbursement activities before the development of a custom-made reimbursement strategy is futile. We hope that the suggested process above could help companies focus on pursuing those reimbursement activities that could promote their overall reimbursement goals and minimize the waste of time and resources.
We help medical device companies develop and execute reimbursement strategies across multiple healthcare systems and markets worldwide.
Medical Device Reimbursement Strategy FAQs
What is a medical device reimbursement strategy?
A medical device reimbursement strategy is a structured plan that defines how a device will achieve coding, coverage, and payment by aligning evidence generation and commercialization activities with the correct decision makers.
Why is developing a reimbursement strategy early important?
Early strategy development avoids pursuing the wrong reimbursement pathways and reduces wasted time and resources.
Are coding, coverage, and payment always required?
No. In some cases, existing reimbursement mechanisms allow commercialization without payer engagement.
Who are the main reimbursement decision makers?
Decision makers may include healthcare providers, coding authorities, payers, or integrated care organizations.
Can choosing the wrong reimbursement strategy delay commercialization?
Yes. Misaligned strategies often require additional studies or policy changes after market entry.
How can Mediclever help?
Mediclever supports reimbursement landscape analysis, decision-maker identification, and evidence strategy development.